Difference between revisions of "Titration"
(http://en.wikipedia.org/wiki/) |
m (Text replacement - "http://" to "https://") |
||
| Line 2: | Line 2: | ||
The word "titration" comes from the [[Latin]] word titulus, [[meaning]] inscription or title. The French word ''titre'', also from this origin, means rank. | The word "titration" comes from the [[Latin]] word titulus, [[meaning]] inscription or title. The French word ''titre'', also from this origin, means rank. | ||
| − | *[ | + | *[https://en.wikipedia.org/wiki/19th_century 1859] |
| − | Volumetric analysis originated in late 18th-century France. [ | + | Volumetric analysis originated in late 18th-century France. [https://en.wikipedia.org/wiki/Fran%C3%A7ois-Antoine-Henri_Descroizilles François-Antoine-Henri Descroizilles] (fr) developed the first burette (which was similar to a graduated cylinder) in 1791. [https://en.wikipedia.org/wiki/Joseph_Louis_Gay-Lussac Joseph Louis Gay-Lussac] developed an improved version of the burette that included a side arm, and coined the terms "[https://en.wikipedia.org/wiki/Pipette pipette]" and "[https://en.wikipedia.org/wiki/Burette burette]" in an 1824 paper on the standardization of indigo solutions. A major breakthrough in the methodology and popularization of volumetric analysis was due to [https://en.wikipedia.org/wiki/Karl_Friedrich_Mohr Karl Friedrich Mohr], who redesigned the burette by placing a clamp and a tip at the bottom, and wrote the first textbook on the topic, ''Lehrbuch der chemisch-analytischen Titrirmethode (Textbook of analytical-chemical titration methods)'', published in 1855. |
==Definitions== | ==Definitions== | ||
| − | *1: a [[method]] or [[process]] of determining the [[concentration]] of a dissolved substance in terms of the smallest amount of [ | + | *1: a [[method]] or [[process]] of determining the [[concentration]] of a dissolved substance in terms of the smallest amount of [https://en.wikipedia.org/wiki/Reagent reagent] of known concentration required to bring about a given [[effect]] in [[reaction]] with a known volume of the test solution |
==Description== | ==Description== | ||
| − | '''Titration''', also known as ''titrimetry'', is a common [[laboratory]] [[method]] of [ | + | '''Titration''', also known as ''titrimetry'', is a common [[laboratory]] [[method]] of [https://en.wikipedia.org/wiki/Quantitative_research quantitative chemical analysis] that is used to determine the unknown [[concentration]] of an identified [https://en.wikipedia.org/wiki/Analyte analyte]. Since volume measurements play a key role in ''titration'', it is also known as volumetric analysis. A [https://en.wikipedia.org/wiki/Reagent reagent], called the ''titrant'' or ''titrator'' is prepared as a standard solution. A known concentration and volume of ''titrant'' [[reacts]] with a solution of analyte or titrand to determine concentration. |
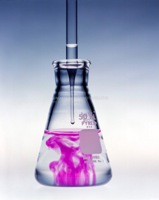
| − | A typical ''titration'' begins with a beaker or [ | + | A typical ''titration'' begins with a beaker or [https://en.wikipedia.org/wiki/Erlenmeyer_flask Erlenmeyer flask] containing a very precise volume of the analyte and a small amount of indicator placed underneath a calibrated [https://en.wikipedia.org/wiki/Burette burette] or [https://en.wikipedia.org/wiki/Pipette chemistry pipetting syringe] containing the titrant. Small volumes of the titrant are then added to the analyte and indicator until the indicator changes, reflecting arrival at the [https://en.wikipedia.org/wiki/Equivalence_point endpoint] of the titration. Depending on the endpoint desired, single drops or less than a single drop of the titrant can make the [[difference]] between a permanent and temporary [[change]] in the indicator. When the endpoint of the reaction is reached, the volume of reactant consumed is measured and used to calculate the [[concentration]] of analyte.[https://en.wikipedia.org/wiki/Titration] |
[[Category: Chemistry]] | [[Category: Chemistry]] | ||
Latest revision as of 02:42, 13 December 2020
The word "titration" comes from the Latin word titulus, meaning inscription or title. The French word titre, also from this origin, means rank.
Volumetric analysis originated in late 18th-century France. François-Antoine-Henri Descroizilles (fr) developed the first burette (which was similar to a graduated cylinder) in 1791. Joseph Louis Gay-Lussac developed an improved version of the burette that included a side arm, and coined the terms "pipette" and "burette" in an 1824 paper on the standardization of indigo solutions. A major breakthrough in the methodology and popularization of volumetric analysis was due to Karl Friedrich Mohr, who redesigned the burette by placing a clamp and a tip at the bottom, and wrote the first textbook on the topic, Lehrbuch der chemisch-analytischen Titrirmethode (Textbook of analytical-chemical titration methods), published in 1855.
Definitions
- 1: a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required to bring about a given effect in reaction with a known volume of the test solution
Description
Titration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte. Since volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the titrant or titrator is prepared as a standard solution. A known concentration and volume of titrant reacts with a solution of analyte or titrand to determine concentration.
A typical titration begins with a beaker or Erlenmeyer flask containing a very precise volume of the analyte and a small amount of indicator placed underneath a calibrated burette or chemistry pipetting syringe containing the titrant. Small volumes of the titrant are then added to the analyte and indicator until the indicator changes, reflecting arrival at the endpoint of the titration. Depending on the endpoint desired, single drops or less than a single drop of the titrant can make the difference between a permanent and temporary change in the indicator. When the endpoint of the reaction is reached, the volume of reactant consumed is measured and used to calculate the concentration of analyte.[1]